Enzymatic approaches to synthesize oligosaccharides offer an alternative to chemical syntheses for the production of homogeneous glycans; however, enzyme-based routes typically require lengthy processes. Now, the design of a water-soluble affinity tag has enabled the automation of multistep enzymatic syntheses of mammalian oligosaccharides.
Genes and proteins have been the main focus of biochemistry for decades, whereas the third major class of biopolymers — glycans consisting of sugars — has been relatively ignored. With the discovery of a myriad of additional roles for carbohydrates in biological systems, ignorance is no longer bliss. However, not only does the heterogeneity of carbohydrates from natural sources complicate studies, but access to structurally well-defined synthetic material is also not as simple as ordering plasmids, DNA primers and peptide libraries. Decades of effort in the cloning and expression of enzymes that can be used to string together single sugars has now finally culminated in a new method to automate the syntheses of key human glycans in milligram amounts, using a newly designed purification tag1.
Enzymes called glycosyltransferases (GTs) connect simple sugars together in natural systems. The idea to use robots to mimic the assembly line process of GTs, which build up more complex eukaryotic glycans on lipids and proteins in the cell component called the Golgi apparatus, is a longstanding dream2. However, separation of the growing sugar chain from other aqueous reaction components, required to drive the reaction forward, has been a challenge. Attachment of sugars to a tree-like structure to increase their size allowed the automated enzymatic synthesis of a tetrasaccharide in low yield, but the required filtration process could only remove the low molecular weight components and not the enzymes themselves from the process3.
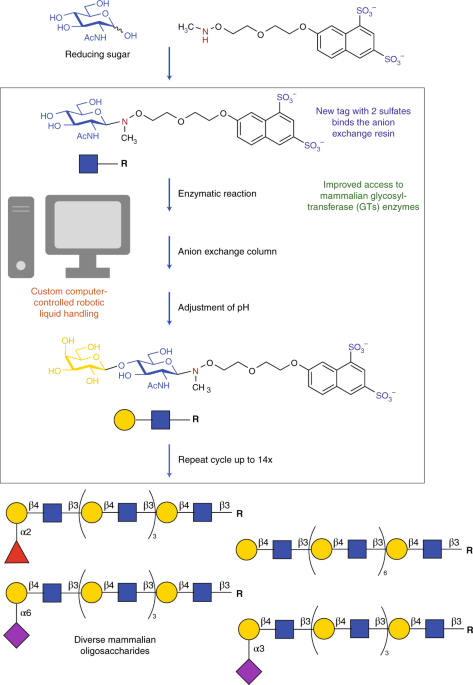
Inspired by other catch and release tags used with solid supports to purify tagged compounds in lieu of filtration, a team led by Geert-Jan Boons developed a series of water-soluble tags containing one to three negatively charged sulfates. They tested the ability of these tags, when attached to sugars, to easily be caught and then released from an anion exchange resin, and discovered a tag with two sulfates that had the best balance of these properties. Now, intermediates can be purified without the need for time-consuming freeze drying steps, which are needed when using aqueous chromatography. This doubly sulfated tag is also compatible with a variety of different enzymes that transfer fucose, sialic acid, galactose and glucose — the common mammalian sugars of O-linked glycans and milk oligosaccharides in humans.
Next, this new tagging strategy was adapted into a custom-designed commercial automation platform similar to those4 used for solution-phase-based automated chemical syntheses of glycans. Fortunately, Boons and co-workers demonstrate that all enzymes maintain enough activity under the sheer forces of the automated liquid handling platform to successfully produce a range of oligosaccharides. Even the lack of availability of any enzymes to transfer mannose was circumvented by tagging a larger precursor glycan isolated from egg yolks and then elaborating this structure to a human N-linked glycan. After serving its role in the computer-controlled automation sequence, the sulfated tag can be removed efficiently with acid, to release the synthetic glycans. Importantly, this process does not also sever the acid-sensitive linkages to sialic acid and fucose. In runs of up to 15 cycles, 12 different mammalian and bacterial enzymes were delivered in controlled sequences to produce three different human milk oligosaccharides and two gangliosides, as well as the one N-linked glycan. Of course, these automation cycles could have been halted at any step to produce many more valuable truncated glycan products.
Interestingly, another team led by Liuqing Wen and Peng G. Wang have simultaneously developed an alternative process for the automation of the enzyme-mediated oligosaccharide process. This process attaches the growing sugar chain to a resin used for the enzymatic glycosylation reactions that is soluble at ambient temperatures, but that becomes insoluble at the 90 °C temperatures used for washing the chain between reaction cycles5. A commercial automated peptide synthesizer with microwave heating is used to implement the iterative cycles of glycosylation. Eight enzymes were successfully delivered to produce five different glycans, including blood group antigens A, B, and O, as well as lactosamine oligomers of up to 12 sugars and a ganglioside glycan, in milligram quantities.
The two demonstrations showing the feasibility of automated enzymatic oligosaccharide synthesis is a boon to everyone clamouring for authentic standards of mammalian O– and N-linked glycans. Both methods should also readily accommodate the use of 13C or deuterium-labelled substrates. Automation is particularly important to making multistep enzymatic processes for glycan synthesis practical. The enzymatic glycosylation reactions reported in both processes take up to a day each; in contrast to automated chemical glycosylation reactions that take less than an hour6. However, chemical glycosylation methods require more time in the syntheses of the building blocks used to feed the robotic platforms, and automated chemistries for this work are still in the development stage. Both automated enzymatic oligosaccharide synthesis processes rely on glycosyltransferases and sugar nucleotides that are becoming increasingly available commercially. With greater demand for these enzymes and reactants, the costs should decrease. If the automated purification methods prove able to operate efficiently with much higher loadings of GTs and reactants, then reaction times could also decrease significantly Fig. 1.
Known aqueous hydroxylamine chemistry is used to attach the catch and release tag to reducing sugars. The resulting tagged sugar is then transferred to a computer-controlled automation platform with a robotic arm that moves solutions between reactor blocks. The glycosyltransferase reaction components are added to the tagged compound and incubated up to 24 h, then loaded onto an anion exchange column. The automated liquid control delivers a series of rinses to the column to elute off undesired components, including the enzyme itself, prior to adding a solution to release the tagged compound. Acetic acid is then used to adjust the pH for the next reaction to ready the tagged intermediate for the next purification cycle. After removal from the automation platform, trifluoroacetic acid is used to remove the sulfated tag.
The ability to use higher enzyme loadings might also enable the incorporation of non-natural sugar substrates, and thereby lessen the advantage that chemical methods have over biochemical methods in generating diversity. Increasing demand for GTs will also drive research to expand the repertoire of enzymes to make a wider range of linkages, including those found in glycans from plants and microbiomes. The increasing flexibility and decreasing costs of automation for chemistry applications should enable the creative expansion of this new work to accommodate the synthesis of more highly charged glycans such as heparin. Clearly, robots are on their way to taking over both chemical and enzymatic oligosaccharide synthesis (and combinations thereof) to leave humans with more time to dream up experiments with the products of these machines!
(원문: 여기를 클리하세요~)