Gold — long presumed to be an inert metal — has been increasingly shaking this image over the past couple of decades, mostly through electrophilic behaviour. Now, a two-coordinate gold complex has been shown to exhibit nucleophilic reactivity, with the insertion of CO2into its polarized Auδ−–Alδ+ bond.
First introduced in the 1970s by Dieter Seebach and Elias James Corey, the concept of umpolung is a cornerstone in organic chemistry. It describes the polarity inversion of an atom or a functional group, resulting in reactivity with an electronic flow inverse to that normally encountered. Now, using such an umpolung strategy, Jose Goicoechea, Simon Aldridge and co-workers have coaxed a gold complex into displaying nucleophilic reactivity in solution1.
Gold complexes are typically known to be extremely powerful electrophiles, in particular towards C–C π-bonds, thereby promoting a number of useful catalytic transformations (such as the hydroamination of alkynes, alkenes, dienes and allenes). Comparatively, transition metal reactivity of gold (such as oxidative addition, migratory insertion, β-H elimination) has only started to be developed2, and nucleophilic behaviour was virtually unknown before this work. Ground-breaking studies by Martin Jansen and co-workers demonstrated in the 2000s the existence of anionic gold species (aurides) in liquid ammonia3, but no nucleophilic reactivity was reported. Conversely, gold was also found to behave as a Lewis basic metal in a few recently isolated complexes, via the formation of Au···H‒X hydrogen bonds4,5 and Au→Lewis acid interactions6. In addition, Daniela Bezuidenhout and co-workers reported in 2016 a tricoordinate T-shaped gold complex featuring two 1,2,3-triazol-5-ylidenes flanking a carbazolide moiety. Its gold centre was shown to be unusually electron-rich, as illustrated by protonation and alkylation reactions7.
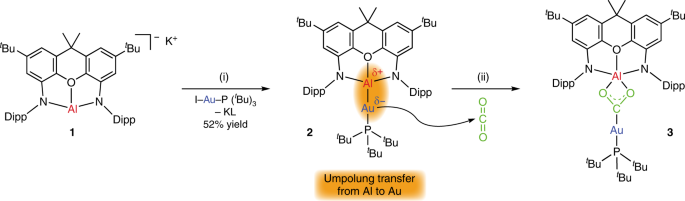
In 2018, Goicoechea, Aldridge and colleagues showed8 that aluminium — a common Lewis acid used in many catalytic transformations — could be converted into a nucleophilic species through smart control of its coordination sphere with a tridentate (N,O,N) ligand (Fig. 1). Compound 1, which exists as a dimer, was made to react with a variety of organic and organometallic electrophiles, enabling the formation of unsupported aluminium–carbon and aluminium–heteroelement bonds.
What this group now shows is a spectacular next step: the aluminyl anion 1 readily binds to gold to form the linear two-coordinate complex 2 (Fig. 1)1. The presence of a bulky phosphine ligand at the gold centre (PtBu3) is required to prevent the formation of a dinuclear species. Complex 2, isolated as colourless crystals, is stable for weeks at room temperature. The Au–Al bond length determined by X-ray diffraction analysis is, at 2.402(3) Å, the shortest yet to be reported. As expected from the very large difference in electronegativity between Au (2.54) and Al (1.61), the Au–Al bond is highly polarized Auδ−–Alδ+. The description of metal–metal bonds involving gold is always a matter of debate, as the very high electronegativity tends to challenge the usual covalent and ionic bonding schemes. Yet calculations leave no doubt that the formation of 2 results from a significant transfer of electrons from Al to Au (by 1.56 electrons according to quantum theory of atoms in molecules (QTAIM) calculations) resulting in partial negative charge at Au (–0.82) and positive charge at Al (+0.56). Thus, the conversion of 1into 2 is accompanied by a transfer of umpolung. In other words, the electron-rich character of aluminium is transferred to gold.
Most remarkable and illustrative of the Auδ−–Alδ+ bond polarity is the reaction of complex 2 with CO2 leading to complex 3. The gold atom binds to the central carbon atom, thus behaving as a nucleophile. In the meantime, the Lewis acidity of the aluminium centre is quenched by coordination of the oxygen atoms, resulting in a formal insertion of CO2into the Au–Al bond of 2. The study also discloses similar reactivity of the Au–Al complex with a carbodiimide, iPrNCNiPr, forming a similar Au–Al insertion product to 2, featuring a Au–C(NiPr)2–Al moiety instead of the Au–C(O)2–Al one. Here, density functional theory calculations would be very welcome to shed light on the mechanism of this unorthodox reaction. Is the addition of the Au–Al bond to CO2concerted? Which pole, the nucleophilic gold centre or the Lewis acidic aluminium centre drives the reaction, if any?
The bonding situation in 2 is reminiscent of that of existing gold–boryl complexes, prepared from boryl anions9. In those, too, the gold centre is electron-rich and the gold–boron bond is polarized as Auδ−–Bδ+, although to a lesser extent. So far, no nucleophilic reactivity has been reported for those gold–boryl complexes, but in light of the behaviour of the related gold–aluminyl complex 2 their reactions with CO2, and heteroallenes in general, are definitely worth exploring.
In any case, the gold–aluminyl complex 2 that has been prepared through an umpolung transfer strategy, and features a polarized Auδ––Alδ+ bond, unambiguously displays nucleophilic reactivity at its gold centre. These findings emphasize the critical role of ancillary ligands in tuning the properties of both transition metals and main-group elements. Gold complexes have found tremendous interest as Lewis acids. Nucleophilic behaviour, as substantiated in this work, opens a new facet in gold chemistry. More generally, these findings underscore the importance of investigating new bonding situations and reactivity paths to advance our knowledge in molecular chemistry.
(원문: 여기를 클릭하세요~)