Finely tuned interactions in the second coordination sphere of enzymes or homogeneous catalysts can be essential for their function. Now, this concept has been applied to the surface of a catalytic material, utilizing pairs of Cu atoms for the selective electrochemical fixation of CO2.
From DNA repair and natural photosynthesis to biological nitrogen fixation, redox-active metalloenzymes enjoy high reactivity thanks to the delicate local environments around their active sites. When electron transfer takes place in such systems, the energetics of both the transition and final states can be stabilized with the transfer of a charged species, usually a proton, through interactions in the second coordination sphere1. Such a synergistic proton-coupled electron transfer leads to low activation energy for catalysis as well as long-range charge transfer in enzymes1. Inspired by nature, chemists seek to develop molecular catalysts with ingenious ligand design to similarly fine-tune the interactions occurring in the second coordination environment. Using characterization techniques such as crystallography, chemists have been able to determine structures and fundamental mechanistic details of enzymes and model catalysts that have enabled them to design kinetically efficient catalysts with high turnover frequencies2,3.
The success of fine-tuning interactions in the second coordination sphere of catalytic molecular systems invites us to wonder whether we can apply this concept to catalytic materials. Two challenges present themselves. The first one involves sample preparation: although molecular catalysts are universally uniform as chemical compounds, a large degree of heterogeneity may exist on a material’s surface. Yet the recent rise of single-atom catalysts4 — which possess individual atoms as active sites distributed on a presumably reaction-innocent surface — provides a platform to study such interactions. Then comes the second challenge: obtaining structural information at the atomic level for catalytic materials so as to establish a structure–function correlation. Thankfully, the development of characterization techniques such as electron microscopy and synchrotron radiation-based X-ray methods has significantly advanced our understanding of materials at the atomic level. Mastering both the synthesis and characterization of single-atom catalysts offers a unique opportunity to study the synergistic effect in the second coordination sphere for heterogeneous catalysis.
Now, writing in Nature Chemistry, Chen and co-workers have described how pairs of Cu atoms on a material’s surface can exhibit a synergistic effect for electrochemical CO2 fixation (Fig. 1)5. Built upon their strong expertise of single-atom catalysts4,6, the researchers developed a method to synthesize Pd10Te3 alloy nanowires loaded with a controllable amount of Cu, ranging between 0 and 0.20 wt%. The extremely low loading amount of Cu ensures sparsely distributed Cu atoms, which is desirable for mechanistic investigation. They tested the catalytic properties of the prepared materials for electrochemical CO2fixation and measured both the selectivity of generating CO from CO2(versus proton reduction to H2) and the rate of CO formation. While Pd10Te3 nanowires are not known to be very reactive, the added Cu is well known for its catalytic activity in CO2 fixation. Thus, one may intuitively postulate that higher loading amounts of Cu should translate to higher values of both selectivity and reaction rate. Instead, an optimal loading amount of Cu was discovered, at roughly 0.10 wt%, which exhibited distinctively high selectivity and reaction rate. This intriguing observation led Chen and colleagues to investigate the underlying mechanism.
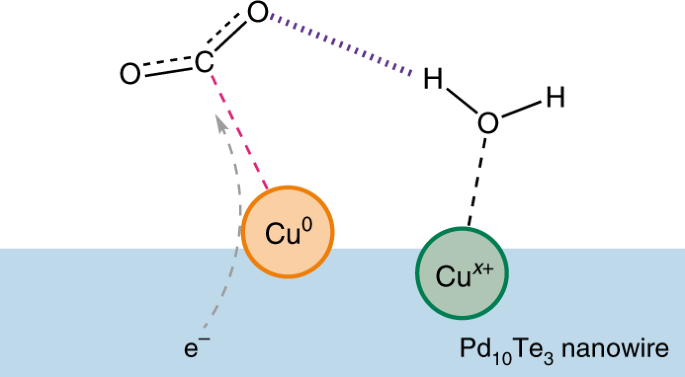
A metallic Cu0 atom activates CO2 and facilitates electron transfer, the product of which may be stabilized by hydrogen bonding from the H2O bound to a proximal Cux+ atom (x = 0, 1, 2). Figure adapted from ref. 5, Springer Nature Ltd.
They characterized the catalysts and obtained detailed structural information using electron microscopy and synchrotron radiation-based X-ray techniques. They concluded that at low loading amounts the surface possesses clusters of four Cu atoms in which a pair of Cu atoms is exposed as the possible active site with two additional atoms sitting underneath. One atom of the exposed pair is metallic (Cu0), whereas the other is less well defined (Cux+, x = 0, 1, 2) (Fig. 1). By contrast, at loading amounts higher than 0.10 wt%, the average oxidation state of the Cu atoms approaches +2 and a Cu species similar to that in bulk CuO is generated. Therefore, Chen and colleagues propose that it is the pairs of exposed Cu atoms (Cu0–Cux+) on the Pd10Te3 nanowires that are predominantly responsible for the observed high reactivity of CO2 fixation.
The Cu0–Cux+ pair is proposed to exhibit a synergistic effect that involves hydrogen bonding. Computational results suggest that the metallic Cu0 acts as an active site for CO2 binding and electron transfer, while the proximal Cux+ acts as a Lewis acid, binding H2O. The proton of the bound H2O in the second coordination sphere of Cu0 is acidic enough to potentially form a hydrogen bond with the terminal oxygen in CO2 and promote the formation of CO (Fig. 1). Comparing with control scenarios, the interaction in Cu0–Cux+ is reported to lower the energy of the transition state during charge transfer. Furthermore, the strong tendency for hydrogen bonding seems to raise the energetics of both hydride formation and the binding of CO, which suppresses the competing proton reduction to H2 as well as the further reduction of CO into other organic species, respectively.
The computational results are consistent with observed high selectivity and reaction rate when the Cu0–Cux+ pair is most abundant, corroborating the claim that the Cu0–Cux+ pair is more reactive and can serve as the basis for advanced design of CO2 reduction catalysts. In the future, additional characterization, especially using in-situ electrochemistry techniques, should provide more information about the catalytic process and help illustrate the proposed mechanism. Also, one feature of the Cu0–Cux+ pair is that the reaction was observed to involve a rate-limiting, single-electron transfer to CO2, which is most likely followed by a subsequent proton transfer. It will be very interesting if the rate-limiting step can be switched to proton transfer or even a concerted process2, with the use of other atom pairs that modulate the redox chemistry, the binding strength of CO2 and the acidity of bound H2O.
Overall, the reported Cu0–Cux+ pair, dubbed by Chen and colleagues as an atom-pair catalyst, showcases a model system that translates the interactions in the second coordination sphere into heterogeneous catalytic materials. The synthetic and characterization methods demonstrated by the researchers can be applied to other catalytic systems and the concept of pairing atoms for a better catalyst will open up new opportunities for atomically dispersed catalysts. When marrying atoms and creating synergy, two are better than one.
(원문: 여기를 클릭하세요~)