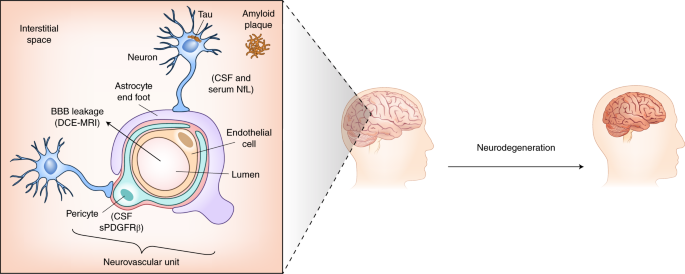
Two new biomarkers for Alzheimer’s disease include one in the blood that relates to neurodegeneration and another that reflects blood–brain barrier dysfunction and is identifiable in cerebrospinal fluid analysis.
Amyloid β (Aβ) plaque and tau tangle pathology are hallmarks of Alzheimer’s disease (AD), the commonest dementia, and each can be measured in vivo using both fluid and imaging biomarkers. In recent years, it has become clear that many additional pathological changes occur in AD that may better predict disease onset and progression and that non-AD pathologies also contribute to cognitive decline. In two separate studies in this issue of Nature Medicine, novel biomarkers for neurodegeneration and blood–brain barrier dysfunction (Fig. 1) are examined in relation to the onset and progression of cognitive decline and AD1,2.
In the capillary neurovascular unit, pericytes, which are capable of shedding PDGFRβ, are located outside the endothelial cells and are separated from them and the parenchyma by a layer of basal lamina. In the parenchyma, astrocyte endfeet and NfL-rich neuronal terminals are closely associated with the capillary. Preische et al.1 find that increased CSF and serum concentrations of NfL reflect early neurodegeneration in AD. Nation et al.2 find that CSF sPDGFRβ is a novel early biomarker for BBB dysfunction, which correlates with BBB breakdown as measured by DCE-MRI. BBB, blood–brain barrier.
Combining longitudinal clinical and population-based cohorts with robust biomarkers that are known to correlate with neuropathology has led to major advances in our understanding of the pathological progression that underpins AD. Accumulation of Aβ in the brain is a very early event, starting at least a decade (and probably longer) before symptoms appear3. Aβ can be measured using two broadly interchangeable biomarkers: the cerebrospinal fluid (CSF) Aβ42/Aβ40 ratio and amyloid positron emission tomography (PET)4. Recently, measurement of CSF has been translated into promising blood tests that will probably be used for early screening for Aβ accumulation to identify subjects for clinical trials to reduce the numbers of invasive tests required to identify eligible individuals4. While Aβ is necessary for an AD diagnosis, it is not sufficient to cause cognitive decline. In parallel with Aβ accumulation, CSF concentrations of total and phosphorylated tau increase5, likely indicating an Aβ-related change in tau metabolism resulting in increased secretion of tau from affected neurons6,7. This tau dysfunction eventually manifests as tangle pathology, which can be visualized using tau PET imaging8, that then becomes neuronal cell loss, i.e., neurodegeneration, which correlates more closely with cognitive decline.
The majority of clinical trials attempting to modify the course of AD have targeted Aβ; although several promising studies are still underway, those completed to date have universally failed to meet their primary cognitive outcomes. Some have shown effects on Aβ pathology, but none has shown robust evidence of an effect on neurodegeneration9. A major challenge has been identification of biomarkers that reflect neurodegeneration and that can predict an individual’s proximity to developing cognitive decline. One such candidate is neurofilament light (NfL), a protein intrinsic to the axonal cytoskeleton. When neurons die, NfL is released into brain interstitial fluid that communicates freely with the CSF as well as with the blood through arachnoid villi and paravascular drainage systems10. CSF and blood NfL concentrations increase across a range of neurodegenerative and neuroinflammatory diseases associated with neuroaxonal injury11, and studies in multiple sclerosis show that NfL concentration normalizes within 6–12 months of start of immunomodulatory treatments11. CSF and blood levels of NfL correlate closely and show the same broad dynamics following acute injury; both peak around 40–70 days post-injury and normalize within 6 months12.
Preische et al.1 report the most extensive study to date on serum NfL dynamics in relation to the onset and progression of AD. Analyzing the multicenter Dominantly Inherited Alzheimer Network (DIAN) longitudinal cohort study, they found that serum NfL concentration was elevated ~6.8 years prior to symptom onset in individuals carrying disease-causing mutations. Measuring within-subject rates of change, they show that mutation carriers have elevated rates of NfL increase even earlier, ~16 years before estimated disease onset. Rates of change in NfL levels were predictive of imaging measures of neurodegeneration and hypometabolism and of change in cognitive scores. Taken together, these data suggest that blood NfL concentration may have utility in clinical trials, providing both a means of determining when to initiate disease-modifying treatment and a relatively noninvasive and cost-effective method for assessing effects on underlying neurodegeneration (although this should take into account an annual age-related increase in NfL concentration of ~3%). Importantly, NfL appears to be a marker of neurodegeneration irrespective of underlying cause11; it may thus be used to diagnose neurodegeneration in a broad range of CNS diseases. However, future studies need to examine whether it is an early biomarker for non-AD neurodegenerative disease. While this probably means that NfL is not useful as a diagnostic test for specific neurological diseases, this protein may be a proximity marker (i.e., a marker that reveals how close a patient is to enter a certain stage of a disease) and outcome measure for trials of many neurodegenerative diseases as well as clinical trials using combinatorial therapies. These combinatorial therapies are currently being considered for sporadic AD, the clinical phenotype of which may be modified by a combination of tau, Aβ, TDP-43, α-synuclein and vascular pathologies.
Aside from deposition of misfolded proteins, a number of lines of evidence suggest that dysfunction at the interface between the neuron and its vascular supply—the so-called neurovascular unit—has an important role to play in late-life cognitive impairment. Until recently, there has been a paucity of biomarkers with which to investigate this concept in vivo. Nation et al.2 developed a CSF test for the shedded form of platelet-derived growth factor receptor-β (sPDGFRβ), a protein highly expressed in brain capillary pericytes. The CSF concentration of this protein was measured in individuals who were cognitively normal or showed early AD-related cognitive dysfunction, and the results were related to regional blood–brain barrier permeability measured using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). CSF sPDGFRβ closely correlated with DCE-MRI evidence of blood–brain barrier dysfunction, particularly in the hippocampus, and was increased in individuals with incipient cognitive dysfunction independent of Aβ and tau, assessed using CSF biomarkers (and amyloid PET in a subset of individulas). In addition to a lack of association with classical AD markers, there was no simple correlation with conventional vascular risk factors, suggesting that predisposition to blood–brain barrier dysfunction—the cause of which is currently unknown—may be an independent risk factor for cognitive decline.
If blood–brain barrier breakdown is a risk for, or cause of, cognitive dysfunction, could this explain increased serum NfL concentration in AD? The lack of association between AD pathology and CSF sPDGFRβ mitigates against this being the whole explanation, although sPDGFRβ concentration may reflect only some aspects of dysfunction of the blood–brain barrier. Future studies combining measurement of NfL, Aβ, tau, and emerging biomarkers of blood–brain barrier dysfunction have the potential both to explain not only the mechanism by which NfL enters the blood, but also more fundamental questions concerning the relationship between, and relative contributions of, vascular dysfunction, AD pathology and neurodegeneration to late-life cognitive impairment. In the more immediate term, NfL is emerging as a dynamic fluid-based biomarker for neurodegeneration irrespective of primary cause(s) and is likely to find utility as an outcome measure (and possibly proximity marker) for a range of clinical trials aiming to slow the neurodegenerative process.
(원문: 여기를 클릭하세요~)