Abstract
The 1989 claim of ‘cold fusion’ was publicly heralded as the future of clean energy generation. However, subsequent failures to reproduce the effect heightened scepticism of this claim in the academic community, and effectively led to the disqualification of the subject from further study. Motivated by the possibility that such judgement might have been premature, we embarked on a multi-institution programme to re-evaluate cold fusion to a high standard of scientific rigour. Here we describe our efforts, which have yet to yield any evidence of such an effect. Nonetheless, a by-product of our investigations has been to provide new insights into highly hydrided metals and low-energy nuclear reactions, and we contend that there remains much interesting science to be done in this underexplored parameter space.
Main
An extraordinary scientific claim was announced on 23 March 1989: the thermal energy produced during electrolysis of heavy water using a palladium cathode exceeded the energy accounted for by the input electricity and all known chemical processes1. This potentially novel nuclear effect became known as ‘cold fusion’. It offered the prospect of clean, abundant, inexpensive energy, and therefore generated global media attention2,3,4. But the generation of anomalous heat or nuclear fusion products during electrolysis was not appropriately validated, and the claims were swiftly dismissed by the scientific community5,6,7,8. In November 1989, a panel convened by the US Department of Energy recommended against any special funding for the investigation of phenomena attributed to cold fusion9. In the parlance of police procedurals, the case went cold.
Many graduate students and postdoctoral researchers today have never even heard of cold fusion, yet the term still elicits strong responses from those who experienced the events of 1989. The subject remains effectively disqualified from mainstream academic research. Nevertheless, light-ion fusion does not violate the conservation of energy, so one cannot completely reject the possibility (however remote) that the clever use of chemistry and materials science could access such phenomena. For this reason, a small subset of the scientific community has remained, to some extent, open to the idea of cold fusion. Isolated groups have continued its pursuit, but have yet to produce a credible ‘reference experiment’ that provides unambiguous evidence of anomalous heat or nuclear reaction products that can be independently verified and advanced.
We came together in 2015 to determine how to produce reliable and accessible experimental data to better inform the polarizing debate about cold fusion that has simmered for three decades10,11,12,13,14,15,16. Our effort comprised approximately 30 graduate students, postdoctoral researchers and staff scientists. It was conducted in accordance with two guiding principles: transparent access to all researchers, apparatus and data; and stringent internal peer review. The concept of the principal investigators operating as a ‘peer group’ was integral to the programme. The plan was to conduct two years of research beginning in 2016, assemble research teams at several academic laboratories, encourage interaction among the teams, keep a low profile to avoid distraction, and to ultimately publish our results. A key objective of our programme was to define quantitative bounds for the observation of any anomalous thermal or nuclear effects. If credible evidence of an anomaly were found, the apparatus would be developed into a reference experiment that could be vetted by the rest of the peer group and eventually the broader scientific community. This is the first public disclosure of our programme.
So far, we have found no evidence of anomalous effects claimed by proponents of cold fusion that cannot otherwise be explained prosaically. However, our work illuminates the difficulties of producing the conditions under which cold fusion is hypothesized to exist. This result leaves open the possibility that the debunking of cold fusion in 1989 was perhaps premature because the relevant physical and material conditions had not (and indeed have not yet) been credibly realized and thoroughly investigated. Should the phenomenon happen to be real (itself an open question), there may be good technical reasons why proponents of cold fusion have struggled to detect anomalous effects reliably and reproducibly. Continued scepticism of cold fusion is justified, but we contend that additional investigation of the relevant conditions is required before the phenomenon can be ruled out entirely.
We have also learned that studying cold fusion can impact other areas of science and technology. For example, the absorption of hydrogen into palladium is an active area for exploring how metal–solute interactions affect properties relevant to energy storage, catalysis and sensing17,18,19,20,21,22,23,24. This broader impact of our work is a direct result of our need to develop new materials and experimental techniques for advancing our understanding of highly hydrided metals and low-energy nuclear reactions25,26.
We believe that there is exciting new science to be done within the parameter space of cold fusion experiments, and that this is an area worthy of engagement from the broader scientific community, even if the discovery of cold fusion at high enough rates for energy applications does not materialize.
Here we will look back at ‘cold fusion’, provide an overview of our programme, consider our results in terms of the programme’s three main initiatives—highly hydrided metals, calorimetry under extreme conditions and low-energy nuclear reactions—and conclude with a brief call to action.
An historical view
The term ‘cold fusion’ was used as far back as 1956 to describe muon-catalysed fusion27,28, but the label is now inextricably linked to the electrolytic experiments sensationalized in 1989 by Martin Fleischmann and Stanley Pons1. Their experiments were designed to measure the heat that evolved during electrolysis experiments involving palladium (Pd) cathodes immersed in lithiated heavy water (Fig. 1). Fleischmann and Pons used the heat balance of the electrochemical cell as a function of current density and electrode size to deduce the enthalpy of the D2O electrolysis reaction. They claimed that this experiment generated more heat than could be explained by the input electrical energy and all known chemical processes. The magnitude of observed ‘excess heat’ led Fleischmann and Pons, and others, to speculate a nuclear mechanism1,29. The detection of neutrons adjacent to the experiment was presented as evidence to support the conjecture that novel fusion processes had occurred.
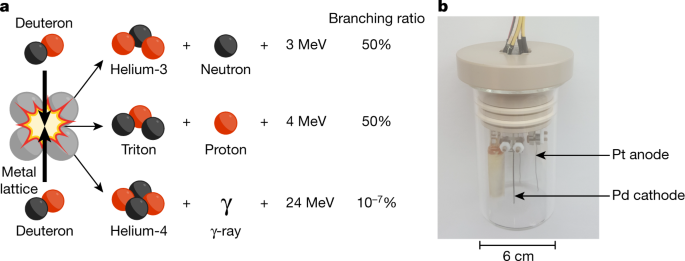
a, Three conventional pathways of deuteron–deuteron (D–D) fusion with their respective products, energy release amounts and branching ratios30. b, Archetypal electrochemical cell for palladium-catalysed electrolysis of D2O.
These results were met with immediate scepticism because nuclei at room temperature should not penetrate the Coulomb barrier at rates appreciable enough to observe fusion. The probability of fusion drops exponentially as particle energy is reduced30,31. Known fusion processes, at rates high enough to be quantified in current experiments, require particle energies greater than about 2 keV in the centre-of-mass frame, corresponding to temperatures greater than 20 million kelvin. However, to this day, disparities remain between predicted and observed fusion reaction rates at low particle energies (in the kiloelectronvolt range), which have been attributed to electron screening effects that enhance the rate of tunnelling through the Coulomb barrier25,26,32,33,34,35,36.
Furthermore, according to the conventional branching ratios for deuteron–deuteron fusion (Fig. 1), far too few neutrons and tritons were detected in the Fleischmann–Pons experiment to account for the quantity of heat observed (a deuteron is a deuterium (D) nucleus, and a triton is a tritium (T) nucleus). It was therefore proposed that D + D → 4He + 24 MeV was the dominant pathway for cold fusion, with essentially all of the energy transferred to the host metal lattice as heat, and helium (the isotope 4He) as the principal nuclear by-product12. Other mechanisms have been proposed as well; however, they tend to violate simple versions of momentum conservation because there is no detectable radiation. A broadly accepted theory that explains cold fusion does not exist at present.
So far, efforts to independently replicate claims of anomalous heat and fusion reaction products have not yielded sufficient evidence to support the existence of cold fusion. In 2004, a second review by the US Department of Energy recommended two areas for additional research to resolve some of the controversies in the field: the material science aspects of deuterated metals using modern characterization techniques; and the study of particles reportedly emitted from deuterated foils using state-of-the-art apparatus and methods37. Few academic laboratories took up this charge. Our programme sought to remedy that situation.
Programme scope
Considerable literature and lore about cold fusion has been produced since 19891,15,29,38 (see also http://lenr-canr.org). Without the guidance of a generally accepted theory, our survey of the field led us to focus on the empirical investigation of three of its most prominent claims: (1) the claim that metal electrodes loaded with extraordinary amounts of hydrogen are a necessary precursor to cold fusion39; (2) the claim that metallic powders heated in a hydrogen environment produce excess heat40; and (3) the claim that pulsed plasma discharges produce tritium and other anomalous nuclear signatures41. It was readily apparent that these experiments would require careful measurement of materials under extreme conditions, including high pressures, temperatures and potentials. Suitable instrumentation would have to be designed, fabricated and calibrated. A few years, not just a few months, were going to be necessary to construct the requisite apparatus and conduct statistically significant numbers of experiments, even within the limited scope of our programme. And finally, we were going to conduct all experiments with a dual purpose: to advance academic understanding of the phenomena under investigation while also evaluating cold fusion claims.
Highly hydrided metals
Michael McKubre and colleagues at SRI International (California, USA) conducted one of the largest studies of cold fusion39. In the early 1990s, they performed dozens of Fleischmann–Pons type electrolysis experiments and claimed to observe excess heat only when the palladium cathode was loaded with hydrogen beyond a threshold of PdHx where x > 0.875 (ref. 39). (For the sake of brevity, ‘hydrogen’ in this Perspective represents hydrogen, deuterium, protons, deuterons, or hydride.) Notwithstanding the ongoing debate in the cold fusion community about whether high loading is important per se, or whether it induces important secondary phenomena (structural defects, for example), we determined that understanding how to create, characterize and sustain highly hydrided metals would be a priority for our programme.
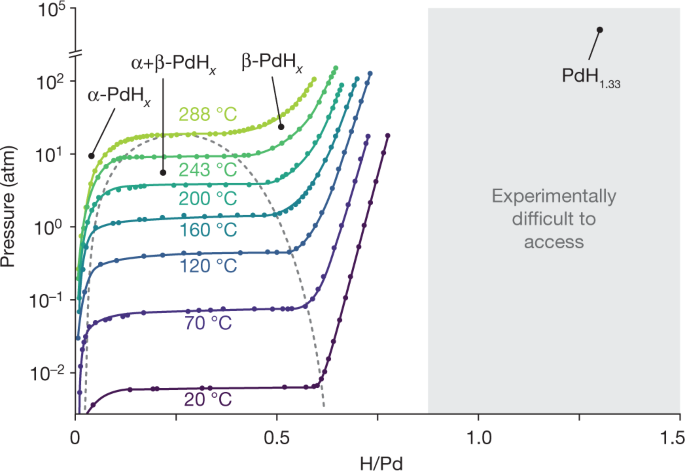
Most Fleischmann–Pons type experiments use palladium cathodes loaded with deuterium, so we focused on the PdHx materials system first. Palladium, one of the few elements that readily absorb hydrogen, forms PdHx where x ≈ 0.7 at room temperature and 1 atm of hydrogen pressure42. However, it is challenging to produce and characterize stoichiometries of PdHx where x > 0.7 (Fig. 2)43,44,45. The absorption of hydrogen by palladium successively forms two distinct phases: a solid solution α-phase at lower hydrogen concentrations, and a lattice-expanded β-phase at higher hydrogen concentrations42. For both phases, hydrogen occupies octahedral interstices in the face-centred cubic palladium crystal lattice. In the β-phase, the composition can conceivably form a rock salt structure with a stoichiometry of PdH1.0. However, at ambient temperature and pressure, the β-phase is limited to a stoichiometry of PdH0.7 (Fig. 2)42; further absorption of hydrogen into β-PdHx requires an exponential increase in hydrogen pressure. There are very few studies that provide convincing evidence for a bulk loading of x > 0.875 in PdHx (refs 46,47,48,49), and even fewer where x ≥ 1 (refs 44,45,50). In the case where x = 1.33 was observed, the lattice underwent a phase change under extreme conditions (973 K and about 50 katm of mechanical pressure) to form a metastable superabundant vacancy phase, as measured by in situ X-ray diffraction45.
Our research programme sought to explore the regime of PdHx where x > 0.875 (grey area), which is not well studied because it is difficult to produce and characterize accurately. PdH1.33 (top right in grey area) has the highest documented hydrogen-to-palladium ratio45. Figure adapted from ref. 42 with permission from Springer. (The pressure–composition–temperature diagram for the palladium–deuterium system does not differ substantially42).
We considered a range of techniques to produce and sustain highly hydrided palladium44,45,51,52,53. Electrochemistry emerged as our method of choice because a modest applied potential produces high hydrogen fugacity, thereby enabling high hydrogen loading without high-pressure hydrogen gas54,55. Merely 120 mV in overpotential produces the thermodynamic equivalent of approximately 100 atm of pressure56, but competing hydrogen desorption reactions still make it difficult to reach exceptionally high hydrogen loading levels. The numerous types of palladium electrodes we tested in aqueous media all yielded values of x < 0.875 in PdHx (ref. 57), except for a single sample where x = 0.96 ± 0.02 (ref. 53). We were able to reproducibly sustain high hydrogen loadings in palladium (x = 0.81 ± 0.02) when we subjected a purpose-built solid-state electrochemical cell to a modest electrochemical driving force of −1 V versus the reversible hydrogen electrode (RHE)53,58. This cell design enabled the use of X-ray diffraction to measure the loading levels during device operation by tracking the lattice expansion upon hydrogen absorption58.
Characterizing hydrogen concentrations in palladium is also challenging. Loading palladium electrodes with interstitial hydrogen causes the metallic lattice to expand, the electrical impedance to change and the mass to increase. We found that in situ X-ray diffraction58, which measures lattice parameter changes, and stripping coulometry59,60, which measures charge passed during hydrogen desorption, to be the most accurate ways of characterizing PdHxstoichiometry in electrochemical environments. We discovered, and corrected, errors in previously used lattice expansion calibrations53 that would otherwise lead to an overestimation of x. In our experience, four-terminal sensing, which measures electrical impedance to infer PdHxstoichiometry, disagreed with X-ray diffraction on the same samples. Mechanical stresses and irreversible plastic deformation caused by lattice expansion affects the impedance of the electrodes independently of hydrogen loading. Finally, to track gas-phase loading of hydrogen in thin metal films, we designed a quartz microbalance that measures mass increase as hydrogen ions are absorbed52. For accurate measurements of hydrogen loading with this technique, additional factors such as stress-induced film curvature during gas absorption must be included52.
Our experience affirms that the materials science aspects of deuterated metals merit further study, as concluded in the 2004 US Department of Energy review37. If loading metals with exceptionally high concentrations of hydrogen is indeed a necessary precursor for cold fusion, then more work is required to produce stable samples of PdHxwhere x ≥ 0.875 to comprehensively evaluate these claims. We also remain intrigued by what properties could arise from PdHx samples where x ≥ 1.
Calorimetry under extreme conditions
Since the early 1990s, researchers in Italy and elsewhere have reported that compositions of certain metallic powders produce excess heat when heated under hydrogen gas40,61. To assess these claims, all of the ways energy can enter, leave, or be stored in an experiment operating at high temperature and high pressure have to be accounted for. Learning how to perform calorimetry under extreme conditions became another priority for our programme.
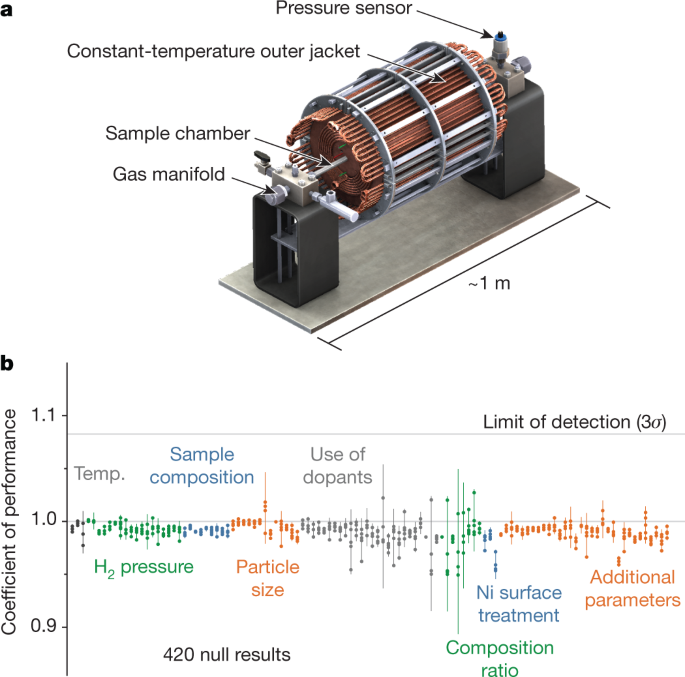
We prototyped several calorimeter designs to enable these studies. This experience acquainted us with many modes of apparatus failure, some quite subtle, induced by the high-temperature, high-pressure environments and the harsh metal/alkali/hydrogen samples required for these experiments (Fig. 3)62,63. We settled on a calorimeter capable of operating up to 1,200 °C and 33 atm with less than 2% measurement uncertainty63. Our design objective was to be able to resolve 10% excess heat to a high degree of confidence. Stated more precisely, we aspired to measure a coefficient of performance (COP, defined as the ratio of energy going out of and into the calorimeter) > 1.09 with 0.98 statistical power, which required replicating each experiment in four identical calorimeters, in accordance with analysis-of-variance principles. (Here, 0.98 statistical power indicates a 98% probability of correctly identifying an effect if there is one.)
a, Rendering of a calorimeter capable of testing for excess heat production at high temperatures and high hydrogen pressures. The calorimeter features a cylindrical alumina sample chamber and 14 independent thermocouple sensors (not visible) within a constant-temperature outer jacket. The ends of the sample chamber are connected to gas manifolds, one of which is equipped with a pressure sensor. b, Plot of coefficient of performance (COP) as a function of the independent variable (shown in coloured text) to evaluate claims of excess heat production by the Ni–H materials system. Each unique experimental condition was typically sampled in quadruplicate. The 3σ limit of detection is presented as a solid grey horizontal line at COP = 1.0825. Dots, 420 individual sample runs; vertical lines, 95% confidence intervals about the average.
Over the course of 16 months, we evaluated contemporary claims of more than 10% excess heat production involving samples of nickel powder and lithium aluminium hydride (LiAlH4). We tested the independent variables of temperature, pressure, sample composition, particle size, surface treatment, and others. To verify the stability of our calorimeters, control experiments were conducted before and after each sample run. We also developed a system identification framework64 to facilitate modelling the time-dependent heat flows and energy storage processes particular to each calorimetry experiment. However, none of the 420 samples we evaluated provided evidence of excess heat; the COPs measured in our experiments were consistently unity (±0.0825 at 3σ; P. A. Schauer et al., manuscript in preparation). We concede that we might not have tested all of the experimental conditions required to initiate excess heat as claimed, and so we have made our calorimeter design and analytical tools publicly available for those seeking to evaluate this parameter space further63,64,65.
Our studies confirm that conducting calorimetry under extreme conditions is challenging, but not intractable. While we have detected no convincing evidence of excess heat so far, our experience with a number of calorimetry systems gives us confidence that we will know it if we see it.
Low-energy nuclear reactions
Thomas Claytor and colleagues at Los Alamos National Laboratory (New Mexico, USA) reported the production of tritium in low-energy benchtop experiments in the mid-1990s41. They used a pulsed plasma discharge in a deuterium gas environment to drive deuterons into a palladium cathode. Evaluating this claim requires measuring fusion by-products (for example, neutrons, protons, tritons, 3He, 4He, or γ-rays) as a function of energy in a challenging regime. We were keen to incorporate nuclear diagnostics into our programme because nuclear signatures, which integrate over the duration of the experiment and provide insight into reaction mechanisms, provide a useful complement to thermal analysis. The 2004 US Department of Energy review also recommended the study of nuclear particles in cold fusion experiments37.
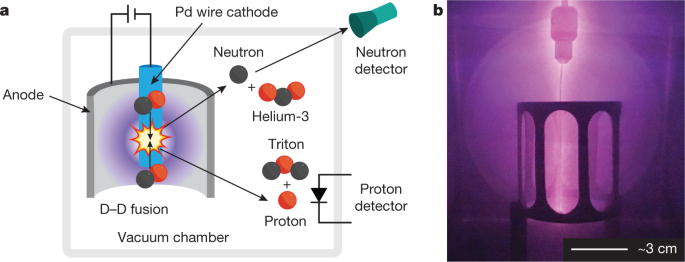
We developed an apparatus to bombard palladium targets with pulsed plasmas of deuterium ions that is capable of producing more flux than the ion beams more commonly used for nuclear astrophysical studies of fusion reactions at low energies25,30,32,33,35,66,67 (Fig. 4). Our initial experiments68 consisted of a palladium wire (cathode) surrounded by a stainless steel cage (anode) housed in a vacuum chamber containing deuterium gas (D2) at about 1 torr. Pulses of electricity (20-µs pulse width, 50-Hz repetition rate, 1-A peak ion current) ionized the D2 and drove D+ ions into the palladium wire. External 3He-based proportional counters and organic scintillators coupled to photomultiplier tubes were used to detect neutrons; an internal silicon diode was used to detect protons.
a, Schematic of the experiment. b, Photograph of the electrodes during the experiment. The conditions claimed to be necessary for fusion are provided by generating a pulsed plasma of deuterium ions between the stainless steel cage and a palladium wire target in a vacuum chamber filled with deuterium gas68.
Early results from these ongoing studies have confirmed that we can produce and detect neutrons from D–D fusion at discharge voltages corresponding to 1.2-keV ion energies in the centre-of-mass frame. The dose rate of deuterium ions in these experiments (1 A cm−2) is much higher than in ion-beam experiments (0.01–0.1 A cm−2)32,35,66,69. Ex situ measurements of the palladium wire targets after prolonged irradiation (hours to weeks, with total fluences of about 1021 D+ cm−2) using scintillation counters have provided no evidence so far of enhanced tritium production68.
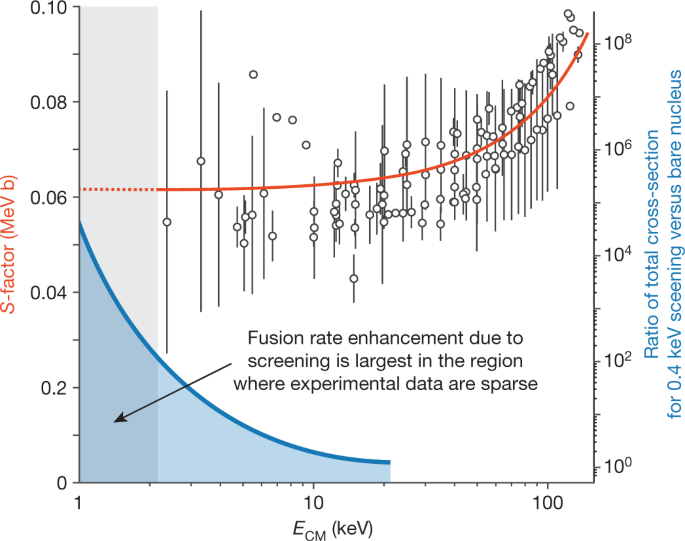
Studying fusion physics at low reaction energies is challenging because the rates of fusion drop exponentially with energy, and quickly approach unmeasurably low levels (Box 1). Accordingly, experimental data for light-ion fusion reactions have large error bars at low energies, and data below 2 keV in the centre-of-mass frame are sparse70 (Fig. 5). The next phase of our plasma discharge experiments will build on our experience of creating and characterizing highly hydrided metal targets. Targets with better controlled materials properties open the possibility of conducting better defined ion-beam30,33,67 and plasma experiments at colder temperatures. We are enthused by the possibility of obtaining reaction cross-section and S-factor (Box 1) data in the grey shaded region of Fig. 5 that could advance the frontier of low-energy fusion physics30,33,66,69,70,71.
Data points show astrophysical S-factor for the D–D fusion reaction (left-hand vertical axis, red) as a function of centre-of-mass energy (ECM); the vertical error bars incorporate both statistical and estimated systematic uncertainties due to initial fuel fractions and measured temperatures. The thick red line shows the best fit extracted from the ENDF/B-VII.1 data library80. The fusion cross-section (σf), and thus the probability of a fusion event, is proportional to the S-factor. The thick blue line shows the ratio of the calculated fusion cross-section for 0.4 keV screening potential (Ue) versus a bare nucleus (right-hand vertical axis, blue) as a function of ECM, with the shaded blue region illustrating the increase in probability of an enhanced fusion rate67. Theoretical predictions suggest that cross-section enhancement due to electronic screening is substantial in the shaded grey region below 2 keV in the centre-of-mass frame, where literature data are sparse and contain large error bars; the dotted red line is the linear extrapolation of the best fit. Figure adapted from ref. 70with permission from Springer.
Fusion stands out as a mechanism with enormous potential to affect how we generate energy. This opportunity has already mobilized a 25 billion dollar international investment to construct ITER72,73. Simultaneous research into alternative forms of fusion, including cold fusion, might present solutions that require shorter timelines or less extensive infrastructure.
A reasonable criticism of our effort may be ‘Why pursue cold fusion when it has not been proven to exist?’. One response is that evaluating cold fusion led our programme to study materials and phenomena that we otherwise might not have considered. We set out looking for cold fusion, and instead benefited contemporary research topics in unexpected ways52,53,57,58,62,63,64,68,74,75,76.
A more direct response to this question, and the underlying motivation of our effort, is that our society is in urgent need of a clean energy breakthrough77. Finding breakthroughs requires risk taking, and we contend that revisiting cold fusion is a risk worth taking.
We hope our journey will inspire others to produce and contribute data in this intriguing parameter space. This is not an all-or-nothing endeavour. Even if we do not find a transformative energy source, this exploration of matter far from equilibrium is likely to have a substantial impact on future energy technologies78,79. It is our perspective that the search for a reference experiment for cold fusion remains a worthy pursuit because the quest to understand and control unusual states of matter is both interesting and important78,79.
(원문: 여기를 클릭하세요~)