The periodic table is immensely powerful for rationalizing many different properties of the chemical elements, but would turning it on its head make some important aspects easier to understand and give everyone a new perspective on chemistry?
It is 150 years since Dmitri Ivanovich Mendeleev first put forward his form of the periodic table, which has been highly successful in terms of arranging the elements correctly and in predicting the existence and properties of elements that were still to be discovered. Since 1869, generations of chemists have proposed variations in format to improve the table, to make it clearer or just to make it more fun1,2,3,4. There are short forms, long forms, spiral forms and even three-dimensional versions reminiscent of Christmas trees. Nevertheless, the traditional ‘Mendeleev’ form has served generations of chemists well, enabling them to rationalize endless different chemical phenomena, with the result that it has survived to the present largely unchanged, apart from having new elements added as and when they were discovered or synthesized.
Think of it, however, from the viewpoint of children sitting at their desks looking for the first time at Mendeleev’s table hanging on the classroom wall. The teacher rarely mentions any of the elements that are typically closest to the children’s eye-level, and talks mostly about those high up near the top of the table. Furthermore, unlike most graphs that plot parameters with values increasing from bottom to top, many properties in the periodic table — atomic number, weight and size of atoms for example — increase from top to bottom.
This is makes it harder to understand one of the key concepts underlying the structure of the periodic table, namely the order of the filling of electron shells. In Mendeleev’s table, these fill from the top to the bottom while most everyday objects (think beakers, baths and waste bins) fill from the bottom up. This contradiction is emphasised by naming this filling the ‘aufbau’ principle which is usually translated as ‘building up’5.
The super-heavy elements are at the bottom and, with the recent confirmation of four new elements, the seventh row of the periodic table has been filled up, and therefore the whole periodic table appears to be ‘complete’. But we believe that many professional chemists still need to look carefully to check which group most of the new elements belong to.
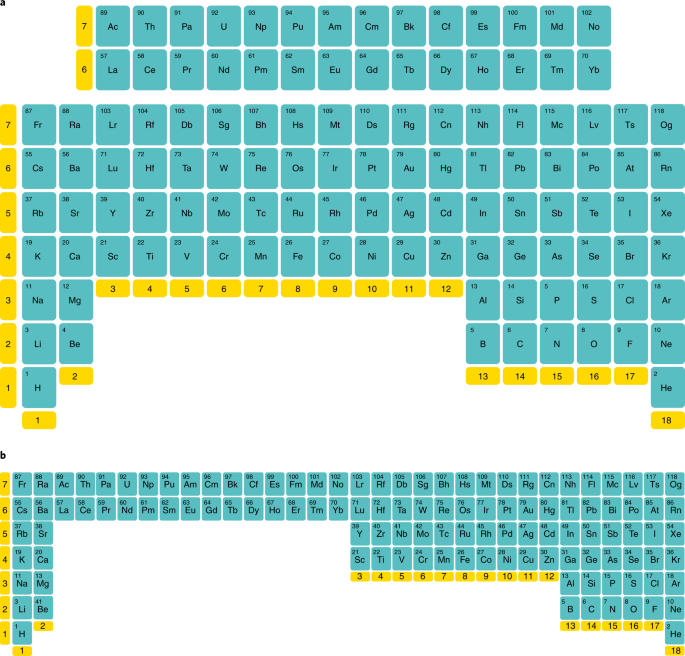
Here we suggest that many of the difficulties described above could be avoided by rotating the periodic table by 180° about a horizontal axis (Fig. 1a) — this puts the light elements at the bottom and the heavy ones at the top. Inverting the table in this fashion has several potential advantages. Notably, most of the properties now increase from bottom to top, including atomic number, atomic mass, atomic radius, maximum oxidation state and reactivity (apart from that of the halogens which are different from the other groups whether the periodic table is in its conventional orientation or in the flipped form). The lighter, more fundamental elements are now at the bottom. So, the aufbau principle becomes more intuitive as the electrons ‘fill up’ the lowest energy orbitals from the bottom, like water in a glass. This is also consistent with psychological evidence that people associate greater magnitudes (numbers) with higher vertical positions6. Despite inversion, each element has all of the same neighbours that it had before, so that none of the traditional relationships between the elements are lost.
a, The lighter elements are now at the bottom and the filling of the electron shells occurs upwards. Just like the traditional representation, many properties (for example, atomic number) increase across the table as one proceeds from left to right, but in the inverted version, the same properties now increase as one moves from the bottom to the top, which is the way that most graphs are plotted. Also like the conventional table, the lanthanides and actinides still sit uncomfortably in an isolated block. b, In principle, this could be overcome by inverting the ‘long form’ of the table but, like the conventional long form, it is probably too elongated to be very useful to most chemists.
We are not aware of an inverted orientation of the periodic table being used previously as a teaching aid. However, after making our original proposal for inversion, we came across an informal online exchange7which suggests that the inverted form may be more intuitive: “I’m reading ‘The Disappearing Spoon’ (actually listening to it on an Audiobook) and, in order to see what the hell the author is talking about, I printed out a copy of the Periodic Table. It seems to me that it’s upside down. It would make much more sense if it showed the elements proceeding upwards as they gathered atomic weight and complexity. Yes? No? Joe (I’m all ears) Nation”.
We have not yet tested this inverted periodic table in either a high school or public environment, but we have shown our table informally to a number of professional chemists including a member of the relevant IUPAC committee. Given that the renumbering of the groups in the periodic table 30 years ago (changing from the A/B notation to simple 1 to 18) caused a widespread outcry amongst chemists, we were surprised that our admittedly limited sample of chemists were almost uniformly positive about the inversion which is clearly more dramatic a change than renumbering. One person commented “You have finally given the periodic table legs!” Two people said that the periodic table is a list and, like shopping or laundry lists, should read from top to bottom. This seems a poor analogy, however, because as a list of elements, the normal periodic table reads from left to right rather than top to bottom.
Two more chemists compared the table to a piece of text which, again, is read left to right and top to bottom. We believe that a much better analogy is to a map; the periodic table shows where the different elements are relative to one another like towns on a map. And we use different types of map of the same area to highlight different aspects such as roads, elevation, vegetation and so on. Indeed, some maps in Australia have ‘South’ at the top and ‘North’ at the bottom, but the relative position of towns are unchanged. One reviewer of this article pointed out that, in the inverted form, the heaviest elements are somewhat counterintuitively at the top. However, those elements are also those with the shortest lives, which might logically place them at the top.
Two other people who were shown our inverted table pointed out that the actinides and lanthanides are still in a slightly inelegant position, but now at the top rather than the bottom of the table. This was not, however, a problem for the actinide chemist whom we consulted! This criticism could easily be countered, however, by inverting the long form of the table (Fig. 1b), but the resulting inverted table suffers from the same problems as the current long form; it is so elongated that horizontal relationships are not easy to see and its aspect ratio makes it awkward to print on anything other than bookmarks! In fact, the actinides and lanthanides usually suffer a fate similar to inconveniently located off-shore islands in conventional maps; they get relegated to a separate box wherever there would otherwise be an empty space on the page.
This generally positive reaction prompted us to test people’s perception of the table in a more rigorous manner by collaborating with experimental psychologists. To avoid our results being influenced by preconceptions, we removed all of the lettering from the tables but retained the shape and asked a total of 24 non-chemist undergraduate students, postgraduates and staff to view the patterns on a computer screen and rate them, while we tracked the position of their eyes (see Supplementary Information for detailed methodology and results). This enabled us to test whether the traditional upright or inverted orientation (1) was more liked, (2) appeared more symmetrical and (3) influenced where people tended to look.
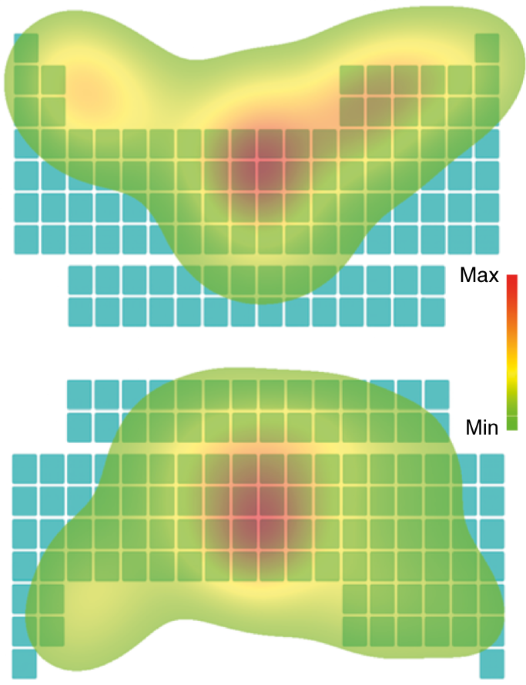
Overall, we found that participants had a modest preference for the traditional orientation over the inverted orientation (4.2 points on a 0–100 scale, p = 0.030). This was despite only six of the 24 participants recognizing it as the periodic table. In scientific aesthetics, a ‘mere exposure effect’ is well known: people like previously seen things more than the novel things, even in the absence of conscious recollection8. People also like abstract symmetry9 but this cannot explain the modest preference for the conventional orientation, because the same participants rated the inverted orientation as slightly more symmetrical. The eye tracking experiments produced a rather more striking result. In both orientations, participants spend the majority of their time looking in the centre. However, their eyes were drawn upwards by the traditional orientation, whereas in the inverted orientation, their eyes were drawn downwards, albeit to a lesser extent (Fig. 2). So, inverting the periodic table inverts how people look at the image, though of course their behaviour might be different if the lettering were in place.
The maps are averaged over 24 participants, four trials per participant; the increasing length of gaze is colour coded green (shortest time) to red (longest time). The scales are marginally different for the two maps (the maximum time is 465 ms in the conventional and 508 ms in the inverted orientation). For both orientations, participants looked for longest in the centre but, in both cases, their eyes were also drawn to the areas corresponding to the lighter elements (see Supplementary Information for a more detailed discussion).
In summary, our proposal to invert the Periodic Table has had a surprisingly positive reception so far, and our tests have not uncovered any obvious drawbacks. Although our collaboration with experimental psychologists revealed a weak preference for the conventional orientation, this could be explained by familiarity, and would thus be reversible. Francl has set the standard for judging periodic tables4“Aesthetics matter, but it always takes a back seat to clarity: any features should be meaningful.”
So, we are not claiming that our version is in any way ‘more correct’ than the traditional table, but we feel that it could have three possible advantages: first, it may make the aufbau principle easier to understand, thereby enthusing more young people to study chemistry; second, looking at a problem from a new viewpoint often gives rise to new ideas, so this orientation of the table will undoubtedly give us all a new perspective; and third, with UNESCO declaring 2019 to be the International Year of the Periodic Table (https://www.iypt2019.org/), it is important for chemists to demonstrate that the table is constantly evolving to meet new challenges.
More generally, it may be wise for us as chemists to think about the diagrams that we use to convey information in chemistry. Are they the best for their purpose, for example for teaching symmetry of molecules or summarizing ever increasing volumes of data? But that’s a task for another day. More immediately, the inverted periodic table should be tested on a wider scale and, to this end, we have included a high-resolution version in the Supplementary Information for you to print out. We encourage you to try it for yourselves.
(원문: 여기를 클릭하세요~)