The ability to synthesize metal-doped nanoplastic opens windows to accurately assess the potential environmental hazards that nanoplastic poses.
Nanoplastic is one of the least studied types of marine litter but potentially one of the most hazardous. This is one of the main outcomes of a recent report1 provided by an expert group advising the European Commission — the Science Advice for Policy by European Academies (SAPEA). The hazard relates to observations that exposure to high doses of nanoplastics causes them to be taken up in the gut of animals by endocytosis or phagocytosis, taken up into the circulatory system of molluscs or taken up in fish1,2,3,4. In our environment, nanoplastic is predominantly formed by fragmentation of larger plastic debris, which means that number concentrations are expected to be high. A probably much smaller fraction of nanoplastic occurring in the environment originated as an added component in some products, for instance those used in facial cleansers5.
No formally accepted or consensus definition of nanoplastic exists, although there is a growing tendency to declare nanoplastics to be a size range smaller than a micrometre. When it comes to definitions, nanoplastic differs from microplastic only with respect to size. Microplastic refers to polymer particles smaller than 5 mm, again through years of habit on the part of researchers rather than by formal definition. The real difference emerges when it comes to detection and analysis. Although it can be challenging, microplastic particles can be isolated from environmental samples by filtration, often in combination with density separation. Things are much more complicated for nanoplastics. Two studies from the past two years propose the use of pre-concentration techniques such as crossflow ultrafiltration in combination with field-flow fractionation and pyrolysis gas chromatography/mass spectrometry6,7. But these techniques are laborious and not applicable to environmental samples with more difficult background matrices, like biota or sediment; it is questionable whether such approaches will ever find application in the detection of nanoplastic in nature.
Because no routine detection method exists for nanoplastic, and no data on environmental concentrations are available, we can only make plausible hypotheses on the environmental fate of such particles — hypotheses that now need verification, because the possibly high number concentrations combined with the bioaccumulation and toxicity profile and the ability of plastic to sorb hazardous organic chemicals8 have led to serious concerns about environmental risks1,3.
Now, writing in Nature Nanotechnology, Denise Mitrano and co-workers propose a tracing method based on nanoplastic particles doped with a chemically entrapped precious metal9. The metal is chosen to be different from the metals present in the background matrix, such that the measured metal concentration is a measure of the metal-doped nanoparticles. Because the outer surface is all plastic, the metal would not interfere with the detection of adverse nanoplastic effects on biota. Furthermore, the quantity of metal is small enough to keep the density of each particle similar to that of particles that can be found in the environment. Yet it is high enough to allow for fast and sensitive quantification, for instance with inductively coupled plasma mass spectrometry (ICP-MS)9. Finally, the particles can be synthesized to obtain either a smooth or more rugged ‘raspberry-like’ surface, thereby accounting to some extent for the hypothesized variability in surface properties of the nanoplastics that would occur in nature.
Although methods to assess the environmental concentrations and exposure to ‘real’ environmental nanoplastics need to progress further, metal-doped nanoplastic could become the first choice for laboratory studies for a wide range of biota, or field studies that aim to detect effect thresholds in ecologically relevant conditions such as those at the level of communities or foodwebs.
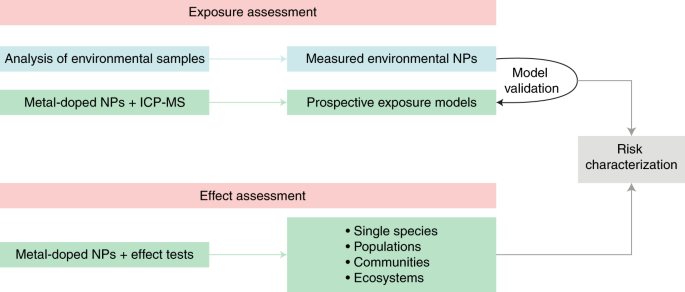
Metal-doped nanoplastics can also be used for mechanistic fate studies on the scale of micro- or mesocosms. As an example, Mitrano et al.9have studied heteroaggregation with natural solids and the fate of the particles within the context of water treatment. Heteroaggregation of nanoplastic is likely to be a key process governing the actual bioavailability of the particles to biota, including humans. This means that aggregation and transport studies with suspended solids and different species of phytoplankton performed with different water chemistries will provide important new insights into the implications of nanoplastics. Such process descriptions (Fig. 1) will yield calibrated parameters that can be used in particle transport models, enabling prospective exposure and risk assessments.
Environmental exposure is assessed based on prospective modelling of nanoplastic concentrations, which in turn is based on process descriptions that use parameters calibrated on experimental data obtained with metal-doped nanoplastics (upper green box). Over time, such models may be validated using retrospectively assessed environmental concentrations obtained from the isolation and instrumental analysis of nanoplastics from environmental samples (blue box). Effect thresholds are assessed for metal-doped nanoplastics as proxies for environmental nanoplastics, from dose–response relationships measured in indoor and outdoor mesocosm experiments using biological endpoints ranging from the level of single species to that of communities and food webs (lower green box). Risk is characterized by comparing exposure and effect threshold data (grey box). NP, nanoplastic.
There is no doubt that this new technique will find a place in many future studies focusing on the ecological effects or environmental fate of nanoplastics.
(원문: 여기를 클릭하세요~)