새로운 형태의 ‘박테리아 세포 내부로 도입된 금 나노물질 기반 에너지 전환 시스템’에 관한 내용입니다.
(원문: 여기를 클릭하세요~)
Intracellular gold nanoclusters act as photosensitizers, enabling non-photosynthetic bacteria to produce acetic acid from carbon dioxide in a more efficient and durable fashion.
Driven by ever-growing consumption, humankind faces great challenges associated with the fossil energy crisis and the environmental footprint of carbon dioxide emission1,2,3. The biological use of sunlight energy for the synthesis of chemicals from water and carbon dioxide provides inspiring blueprints for replacement sustainable-energy materials and green technologies3. The design of semi-artificial photosynthesis systems based on biological building blocks — either isolated machineries4,5 or whole-cell microorganisms6,7,8 — linked with engineered inorganic nanostructures can produce hybrids with properties beyond those of their individual natural and synthetic components. For instance, Yang and co-workers8previously reported the photosensitization of non-photosynthetic bacteria, Moorella thermoacetica, by cadmium sulfide (CdS) semiconductor nanoparticles deposited on the surface of the cells, enabling the conversion of CO2 to acetic acid. But CdS is toxic to cells and the environment, and electron transfer from the photosensitizer on the M. thermoacetica cell membrane to the enzymes within the cell is sluggish. Now, writing in Nature Nanotechnology9, workers from the same group achieve faster electron transfer and more durable solar CO2fixation by implanting ultrasmall synthetic gold nanoclusters covered with naturally occurring peptide glutathione (Au22(SG)18) into living M. thermoacetica.
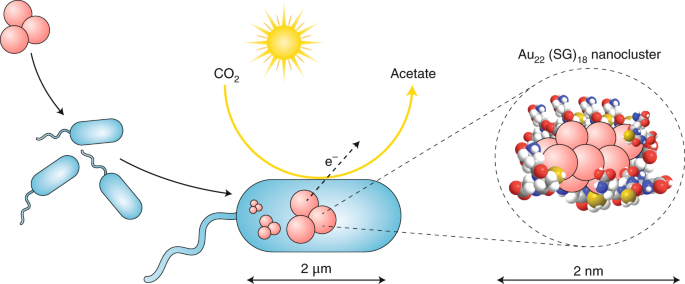
Au22(SG)18 nanoclusters are first synthesized and then added to the cell culture, where they are taken up by the microorganism. The uptake is possibly attributable to the tiny size of the nanoclusters (smaller than 2 nm) and to the biological affinity of glutathione molecules sited on the nanoclusters’ surface towards bacterial walls. Thus, the gold nanoclusters pass through the cell membrane and enter the microorganism’s inner space, flawlessly integrating with the bacteria’s intrinsic route for CO2 fixation, which is known as the Wood–Ljungdahl pathway10, as illustrated in Fig. 1. Biological reduction of CO2 to acetate by M. thermoacetica takes place anaerobically in the dark and uses electrons from hydrogen derived by bacterial enzymes such as hydrogenase. In contrast, the new M. thermoacetica/Au22(SG)18 bacteria engineered by Zhang et al.9 can achieve the same CO2 fixation by using light-excited electrons provided by the implanted gold nanocluster (see Fig. 1). The overall quantum efficiency of the M. thermoacetica/Au22(SG)18 nanocluster biohybrid reaches 2.86 ± 0.38%, outpacing the efficiencies of the biohybrid of M. thermoacetica with surface CdS. Likely reasons are as follows: first, an enhanced interface between intracellular nanostructures and bio-machinery allows more efficient energy transfer from the photo-excited nanocluster to biochemical pathways; next, owing to enhanced physicochemical and photoluminescence properties driven by strong quantum size effects, the Au22(SG)18 nanocluster performs as an excellent light harvester; and finally, the cadmium-free biocompatible approach is benign for the host microorganism, allowing extended longevity and catalytic stability.
The M. thermoacetica/Au22(SG)18 biohybrid is constructed through the uptake of ultrasmall Au22(SG)18 nanoclusters and their consequent implantation into the internal bacterial metabolic pathway. The implanted Au22(SG)18 nanoclusters can be sensitized by visible light, providing the microorganism’s enzymatic machinery with photo-induced electrons for the reduction of CO2 to acetate. In this way, a non-photosynthetic microorganism is turned into an artificial photosynthetic nano–bio factory. A closer view of the nanocluster is shown in the inset, where the surface of the Au22 nanocluster (pink) is ‘wrapped’ by molecules of tripeptide glutathione. Thiolate groups (yellow) in glutathione serve to anchor the peptide ligand to the gold cluster surface and also participate in the light-induced charge separation that is necessary for the catalytic reaction.
Under the anaerobic photosynthetic condition of M. thermoacetica/Au22(SG)18 hybrids, some reactive oxygen species (ROS), such as hydroxyl radical or nitric oxide, are produced, owing to the photo-oxidative degradation of biomolecules under light exposure. These ROS could ‘escape’ from cellular signalling and from deteriorated electron transfer pathways. Interestingly, the researchers find that the Au22(SG)18 cluster can trap harmful radicals9. And they observe, too, that the ROS concentration in the M. thermoacetica/Au22(SG)18 hybrid is 1.8 times lower than in the pristine microorganism after each has been illuminated for 48 hours, indicating that the nanocluster acts as an internal ROS scavenger that enhances the viability of bacterial cells.
Overall, this work is a successful example of a biohybrid system built on delivery of nanoclusters with excellent photochemical, ROS scavenging and biocompatibility characteristics into whole living cells, and their efficient integration into a cell’s metabolic pathway. Not only does this fusing of inorganic and living matter provide insights for improving nano–biohybrid photosynthesis systems, but it also deepens our fundamental understanding of material and energy transfer within biological machinery, and from inorganic material to biological pathways; of biophysical and biochemical interactions; and of quantum size effects and their manifestation at nano–biological boundaries. Since most non-photosynthetic enzyme reactions carried out by whole organisms or isolated biological machineries require energy in the form of cofactors or reducing equivalents, it would be interesting to explore further whether this approach of using light as the energy source can be generalized to other non-photosynthetic microorganisms and other biotechnological processes. Hybrid systems of inorganic materials with microorganisms could be further enhanced by modern synthetic biology — with re-design at various levels, from enzymes, biological circuits and pathways to artificial microorganisms. ‘Big Data’ and modelling to optimize coupling of abiotic and biological matter in a mutually enhancing way can provide an additional thrust that will help new functional hybrids to emerge. However, as material properties often vary within the range of nanoscale sizes because of quantum and surface effects, their biocompatibility, biodistribution and long-term environmental effects should be considered before such nanomaterial-boosted technology is put to practical use.