Immunotherapy approaches seek to boost immune responses against cancer. A single antibody engineered to recognize three targets shows promise, when tested in animals, in improving the ability of T cells to target cancer.
Antibodies with specificity for one target — called monoclonal antibodies — were the first cancer immunotherapy to achieve widespread clinical use. The therapeutic potency of antibodies can be amplified by engineering them to recognize two distinct molecular targets (termed antigens). These bispecific antibodies can simultaneously bind to cancer cells and immune cells called T cells, and this dual binding directs the T cell to unleash its cell-killing power towards the cancer cell. Writing in Nature Cancer, Wu et al.1 now report the development of a trispecific antibody, one that has three targets: a cancer cell, a receptor that activates T cells, and a T-cell protein that promotes long-lasting T-cell activity against the cancer cell (Fig. 1).
Figure 1 | An antibody that helps immune cells to target cancer cells. Wu et al.1 report the development of a human antibody that is engineered to bring an immune cell called a T cell into close proximity with a type of cancer cell called a myeloma cell and to boost the T cell’s anticancer response. This trispecific antibody binds three targets: the protein CD38 on a myeloma cell, and the protein CD28 and the protein complex CD3 on a T cell (the antibody’s target-binding domains are shown in red, blue and yellow, respectively). CD3 is part of the T-cell receptor (TCR), which recognizes abnormal cells by binding molecules called antigens. The binding of CD3 by the antibody drives T-cell activation (without requiring antigen recognition by the TCR), which leads to the killing of the myeloma cell and the production and release of toxic cytokine molecules. Binding of CD28 by the antibody drives expression of the protein Bcl-xL. Bcl-xL blocks T-cell death, which might otherwise occur if there was prolonged TCR activation in the absence of CD28 stimulation by the antibody.
The mammalian immune system generates an immense diversity of antibodies, and antibodies can also be engineered to recognize therapeutic targets. Antibodies usually recognize a single antigen, which might be part of a disease-causing agent or an abnormal version of a protein or sugar. Such monospecific antibodies against targets on cancer cells can recruit immune cells, including neutrophils, natural killer cells and macrophages, to kill or ingest the cancer cells.
Antibodies can also be engineered to block or stimulate the function of the proteins to which they bind. For example, there are regulatory receptors that inhibit T-cell function, and antibodies that have been engineered to block these receptors provide a clinical strategy known as checkpoint blockade, which boosts T-cell function. These inhibitory receptors govern T-cell exhaustion, a non-functional T-cell state that protects against autoimmunity and that can occur in the tumour microenvironment as cancers evade antitumour responses mediated by T cells. Checkpoint-blockade treatment can awaken exhausted antitumour T cells to great clinical benefit, but it also risks causing autoimmune toxicity. The antibody developed by Wu and colleagues takes a similar approach to promote T-cell activity against cancer cells. However, their method stimulates the function of receptors that positively boost T-cell function, rather than blocking the function of inhibitory receptors.
The human antibody developed by Wu et al. builds on bispecific-antibody technology that reconfigures the antigen-recognition domains of two different antibodies into one bispecific molecule. Bispecific antibodies usually target one antigen on the cancer cell’s surface and one on a protein complex on T cells called CD3. CD3 is part of the T-cell receptor (TCR) complex. The TCR also includes antigen-recognition domains and delivers an activating signal to the T cell when an antigen binds. Engagement of CD3 by the antibody also generates an activating signal. Such a bispecific antibody therefore activates T cells, brings them into close proximity to cancer cells — irrespective of the T cell’s natural antigen specificity — and redirects their killing capabilities towards the cancer cells.
This concept has proved to be clinically effective for the bispecific antibody blinatumomab, which targets CD3 and the protein CD19 on cancer cells. Blinatumomab treatment doubles the remission rate and survival among people with an advanced stage of a cancer called B-cell acute lymphoblastic leukaemia (B-ALL)2, and it is being tested as part of the initial therapy for B-ALL, with promising early results3.
Wu and colleagues devised a clever strategy to simultaneously boost T-cell activation and enhance the targeting of cancer cells in relation to multiple myeloma, which is a cancer of plasma cells in the blood. The authors developed a trispecific antibody that was engineered to have three antigen-binding sites, rather than two. This trispecific antibody targets CD3 plus the proteins CD38 (on cancer cells) and CD28 (on T cells). The CD38-targeting antibody daratumumab is clinically effective in treating this disease4, and CD38 is also a potential target in other cancers, such as acute lymphoid leukaemia and acute myeloid leukaemia.
CD28 belongs to a class of protein called co-stimulatory receptors, which positively regulate T-cell activation. When a T cell recognizes its target antigen through the TCR, the extra engagement of a co-stimulatory receptor such as CD28 is needed to achieve the sustained T-cell proliferation required for an effective immune response. In the absence of co-stimulation, activation through the TCR can lead to a state of T-cell non-responsiveness called anergy, or to the related state of exhaustion. Prolonged activation of the TCR without co-stimulation can lead the T cell to undergo a form of programmed cell death called apoptosis.
The addition of a co-stimulatory signal such as CD28 is notable because this signal has also been incorporated into another type of immunotherapy called chimaeric-antigen receptor T cell (CAR‑T) therapy5, in which a receptor is engineered to both recognize a cancer-cell antigen and include T-cell activation domains such as CD3 and CD28. The main reason for including a CD28-binding domain in the trispecific antibody is T-cell co-stimulation. However, CD28 is also frequently expressed by multiple myeloma cells, so this might increase the antibody’s affinity for the myeloma cells, and thus enable it to bind to cells in which CD38 is low, absent or masked by previous daratumumab therapy.
To confirm that the CD28-binding domain augmented the trispecific antibody’s activity, the authors made versions of the antibody in which different combinations of the three binding domains were mutated. They tested these versions in ‘humanized’ model mice, which had human T cells and human myeloma cells. A functional CD28-targeting domain boosted T-cell activation above that observed using antibodies lacking this domain. This augmented T-cell activation drove T-cell proliferation and the expression of the anti-apoptotic protein Bcl-xL in T cells, supporting the authors’ hypothesis that having a co-stimulatory signal would prevent T-cell apoptosis. The presence of the CD28-targeting domain on the antibody boosted the ability of T cells to kill different myeloma cell lines in vitro and in the humanized mouse model, even at the lowest antibody dose tested.
The main limitation of this study is that the risk of a side effect called cytokine release syndrome (CRS), which can occur if the immune system is highly stimulated, is unknown. In CRS, the simultaneous activation of many T cells causes excessive release of signalling molecules called cytokines from cells of the immune system, which drives inflammation. CRS can occur with bispecific antibodies and with CAR-T. It typically manifests as fever, but can progress to fatal multi-organ failure in severe cases6.
The authors report cytokine-related toxicities with their trispecific antibody when administered to monkeys by intravenous injection, but toxicity was less if it was delivered under the skin (subcutaneously) instead, leading to a more gradual exposure to the antibody. It is reassuring that the inclusion of the CD28-targeting domain did not lead to overwhelming CRS in these tests. However, a key caveat is that the amount of CD38 in monkeys is much less than in people with multiple myeloma, and the higher amount of CD38, and thus of antibody-mediated T-cell activation, would probably increase the risk of CRS in humans. But in terms of possible negative effects of the antibody on healthy non-cancerous cells, it is reassuring that only transient decreases in the number of normal white blood cells that express CD38, such as lymphocytes and myeloid cells, were observed in monkeys treated with the antibody. Another limitation of the study is that the authors did not assess whether this trispecific antibody format might trigger an immune response against the antibody and cause its rapid destruction.
Targeting cancer using a trispecific antibody is an important conceptual advance, building on previous work by this group7 on a trispecific antibody that targets HIV. For multiple myeloma, fresh therapeutic approaches are needed, because even the most potent emerging therapies, including a CAR-T that targets an antigen called BCMA, are only temporarily effective for most people8–10. A trispecific antibody is a flexible platform that might offer a way to deliver precise combinations of immunomodulatory signals (for example, a co-stimulatory signal and a checkpoint blocker) specifically in the tumour microenvironment, which might be safer and more effective than the systemic administration of combinations of individual, single-specificity immunomodulatory antibodies. Such efforts to make immunotherapy more precise and potent than it is at present might be necessary to broaden the reach of immunotherapy to include the many types of cancer that have so far proved difficult to target.
Nature 575, 450-451 (2019)
(원문: 여기를 클릭하세요~)
아래는 2022년 11월 4일 뉴스입니다~
(원문: 여기를 클릭하세요~)
약 하나로 두 가지 치료효과…제약社 ‘이중항체’에 꽂혔다
서로 다른 두개의 항원 결합
암세포 죽이면서 면역력 강화
J&J 등 차세대 먹거리로 눈독
국내선 에이비엘바이오 ‘눈길’
최근 글로벌 제약업계에 ‘이중항체 붐’이 일고 있습니다. 차세대 항암 기술로 주목받기 시작하면서죠. 지난달 25일 존슨앤드존슨(J&J)은 미국 식품의약국(FDA)으로부터 이중항체 기반의 다발성 골수종 치료제 ‘텍베일리’에 대한 가속승인을 받았습니다. 다발성 골수종은 플라즈마 B세포가 비정상적으로 많아지면서 생기는 혈액암의 일종입니다. 이중항체인 텍베일리는 B세포 성숙항원(BCMA)과 T세포를 활성화하는 CD3 분자에 동시 작용합니다. 미국에서 다발골수종 치료제로 허가받은 첫 번째 이중항체 신약입니다.
개발 중인 이중항체 기반 후보물질의 임상 결과도 긍정적으로 나오면서 글로벌 제약·바이오 기업의 이중항체 시장에 대한 관심이 높아지고 있습니다. 이중항체 기술을 일단 확보하고 나면 다양한 질병에 적용할 수 있어 사업 확장성도 크다는 평가를 받습니다. 국내 이중항체 선두주자는 설립 7년 차인 에이비엘바이오입니다. 세계 6위 제약사 프랑스 사노피에 이중항체 후보물질 ‘ABL301’을 조(兆) 단위에 기술수출하면서 세계적 관심을 받았습니다. ABL301은 파킨슨병 원인 단백질을 차단함과 동시에 약물의 뇌혈관장벽(BBB) 침투를 높여주는 파킨슨병 치료제입니다.
한국바이오협회에 따르면 세계적으로 개발 중인 이중항체 후보물질은 600여 개에 달합니다. FDA, 유럽의약품청(EMA) 승인을 받은 이중항체 의약품은 6개에 불과합니다. 두 곳에서 모두 승인받은 약물은 2개뿐입니다. 시장이 초기 단계인 셈이죠. 그만큼 넘어야 할 기술장벽이 높다는 뜻이기도 합니다. 이상훈 에이비엘바이오 대표는 “독성을 최소화하고 어떻게 기존 내성을 극복하느냐가 핵심”이라고 했습니다.
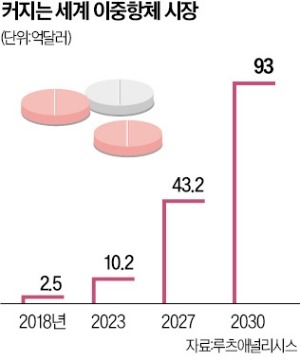
이중항체 시장 전망은 밝습니다. 시장조사기관 루츠애널리시스는 세계 이중항체 시장이 2018년 2억5000만달러(약 3600억원)에서 2030년 93억달러(약 13조원)로 급성장할 것으로 보고 있습니다.
